How To Write The Net Ionic Equation For AgNO3 + NaCl = AgCl...
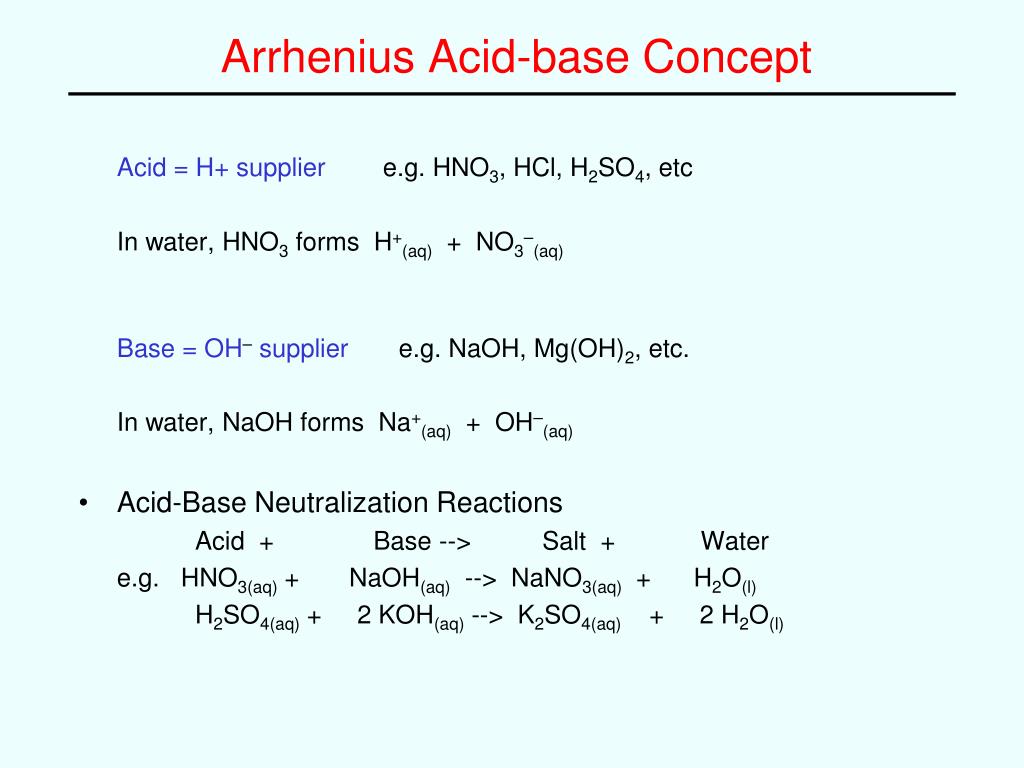
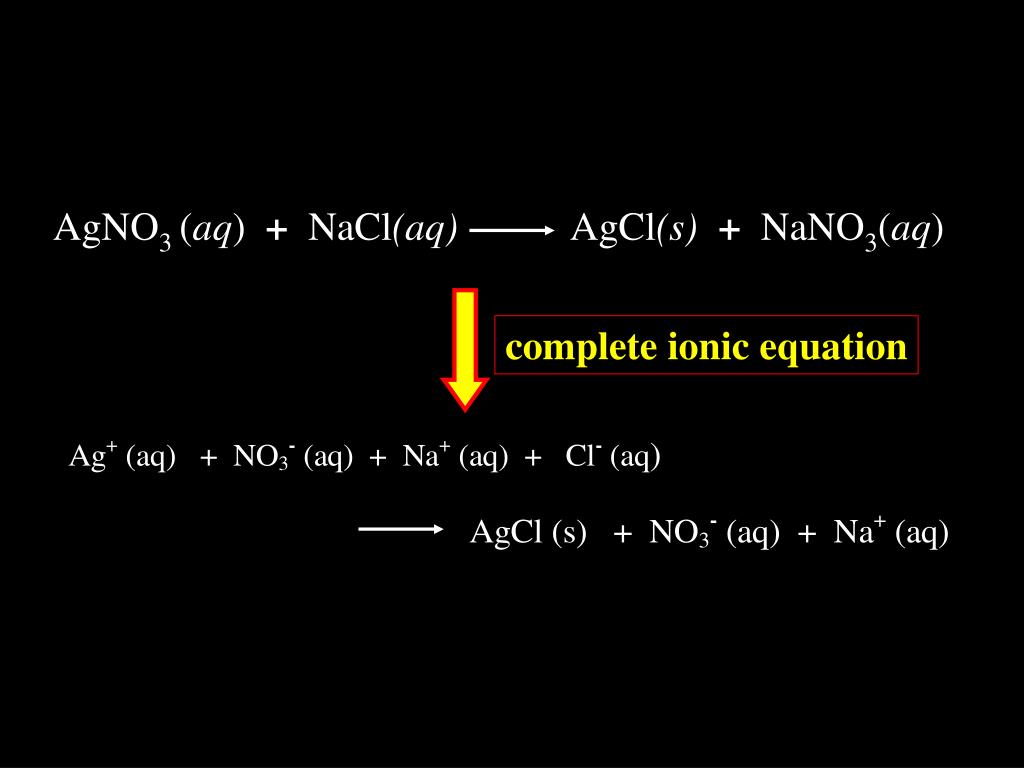
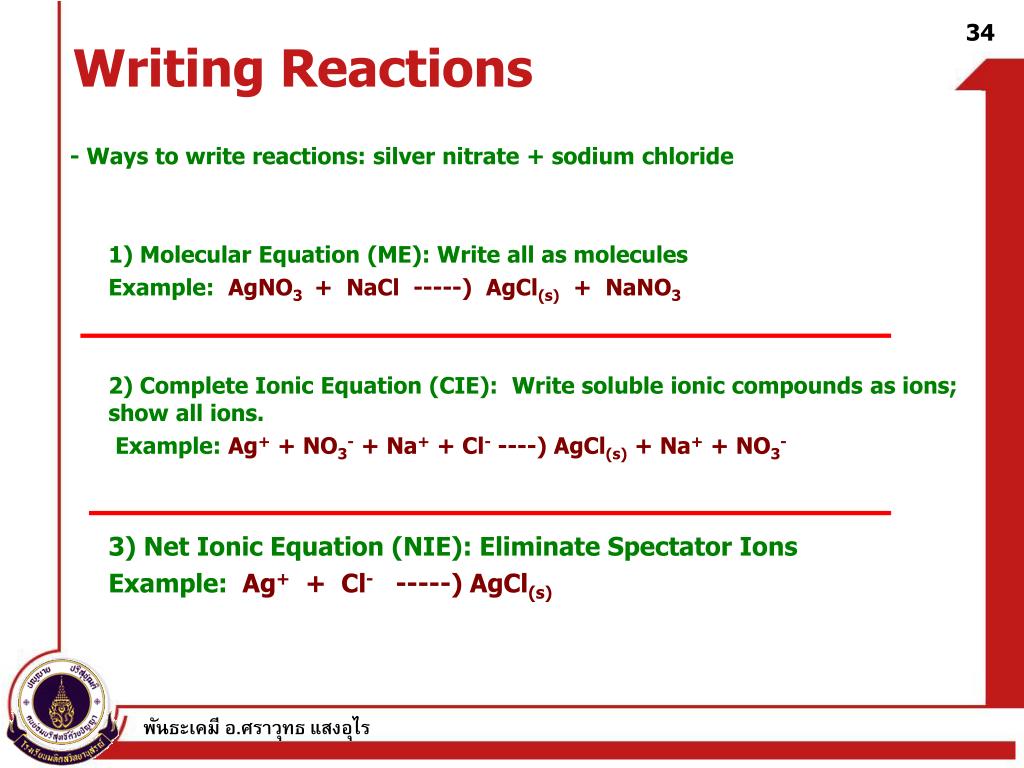
Precipitation reactions as molecular equations, ionic equations and net ionic equations tutorial suitable for chemistry students. Molecular Equation. Consider the reaction between aqueous solutions of sodium chloride, NaCl(aq), and silver nitrate, AgNO3(aq).AgNO3 (aq) + NaCl(aq) AgCl(s) + NaNO3(aq) What happens when you put AgNO3 and NaCl in water? Writing net ionic equations by ewalenta 6799 views.I need help finding the net ionic equation AgNO3(aq) + KCl(aq) ==> AgCl(s) + KNO3(aq) .. molecular equation. You must also know or look up which compounds are soluble (aq) and which are not (s). In this case, silver chloride (AgCl) is not soluble.The net ionic equation for the reaction of AgNO3 with NaCl is Ag+ No3- + Na+ Cl- => AgCl + Na+ No3 Definition: An ionic equation is a chemical equation where the electrolytes in aqueous solution are written as dissociated ions.Examples:Ag+(aq) + NO3-(aq) + Na+(aq) + Cl-(aq) â...A balanced equation results when the number of atoms in the reactants is equal to that in the products. Sodium chloride (NaCl) and silver nitrate (AgNO3) are both solids that are highly soluble in water. They give a double displacement reaction where the ions switch places and give sodium...
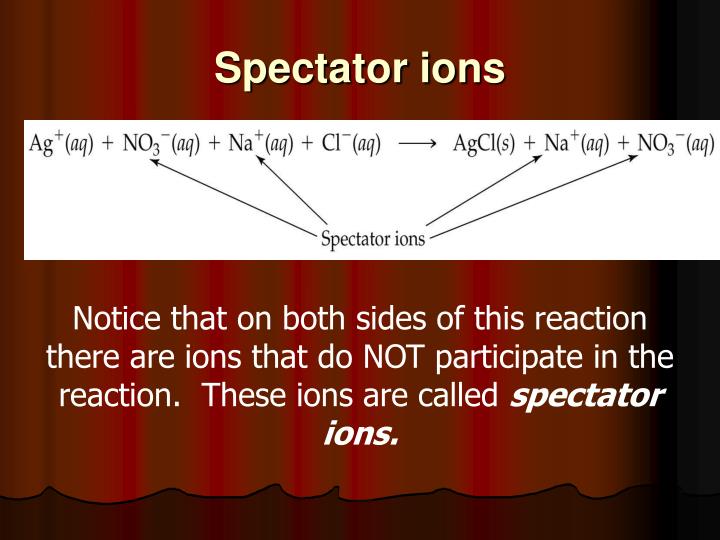
Net ionic equations
Give the equation to represent the autoprotolysis of water?39 AgNO 3 NaCl AgNO 3 NaCl AgNO 3 NaCl AgNO 3 NaCl AgNO 3 NaClAgNO 3 NaCl AgNO 3 NaCl. 54 Total ionic equations Show all dissolved substances (aq) as ions All others (s, l, and g) show as molecular formula Molecular Equation : AgNO 3 (aq) + NaCl(aq) → AgCl(s) + NaNO 3 (aq)...The net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved in the chemical Following reaction is an example of which type of reaction: NaCl + AgNO3 gives AgCl + NaNO3. Since there is an exchange of ions between the...Net ionic equation: Ag+ +Cl- --> AgCl. Volume of (.0100 M) AgNOs to titrate bay water samples: March: 7.3 mL. April: 4.46 mL. 1) Using the balance equation, calculate the average number of moles of NaCl in each of the titrated samples.
Enter the net ionic equation, including phases... | Wyzant Ask An Expert
View all chemical equation transform from AgNO3 (silver nitrate) to AgCl (silver chloride). A precipitate forms in a double-replacement reaction when the cations from one reactant combine to form an insoluble ionic compound with the anions from the other reactant....NaCl --> AgCl + NaNO3 Net ionic equation: Ag+ +Cl- --> AgCl Volume of (.0100 M) AgNOs to titrate bay water samples: March: 7.3 mL April: 4.46 mL 1) Using the balance equation, calculate the average number of moles of NaCl 2) Calculate the average numbr of grams of NaCl in each titrated samples.Your equation is already balanced. AgNO_3(aq) + NaCl(aq) rarr AgCl(s)darr + NaNO_3(aq) Silver chloride will precipitate from solution as a curdy white because of its exceptional insolubility in water. The other materials are along for the ride and will remain in water as the aquated ions, Na^+(aq)...Ionic reactions in aqueous solutions: net ionic equations. Double replacements are among the most common of the simple chemical reactions. Using the solubility table (see below) we find both KNO3 and NaCl are water soluble products.Select the NET ionic equation for the reaction between AgNO3 and NaCl. (The net ionic equation cancels out spectator ions that have the same formula and state of matter on either side of the equation.
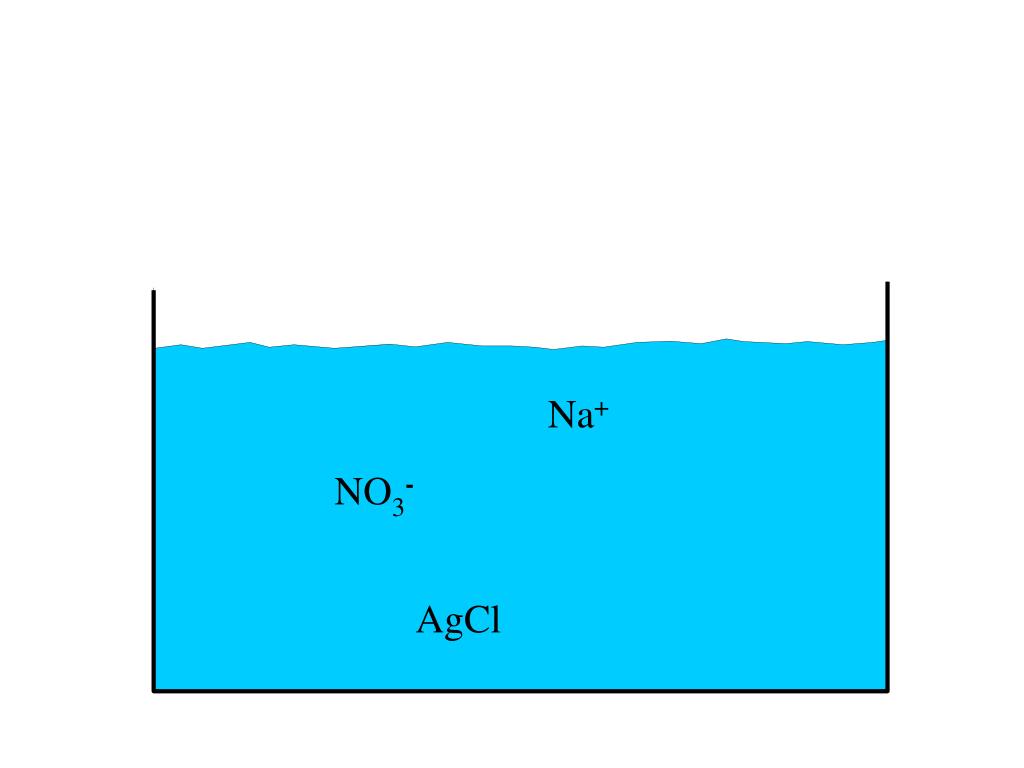
In this double-displacement response, all the states of the reactants are aqueous, that means, they are dissolved in water. The insoluble precipitate, AgCl, is solid. NaNO3 is aqueous as a result of it's soluble in water.
So, we write all the ionic equation with the state symbols as:
AgNO3(aq) + NaCl(aq) ---> AgCl(s) + NaNO3(aq)
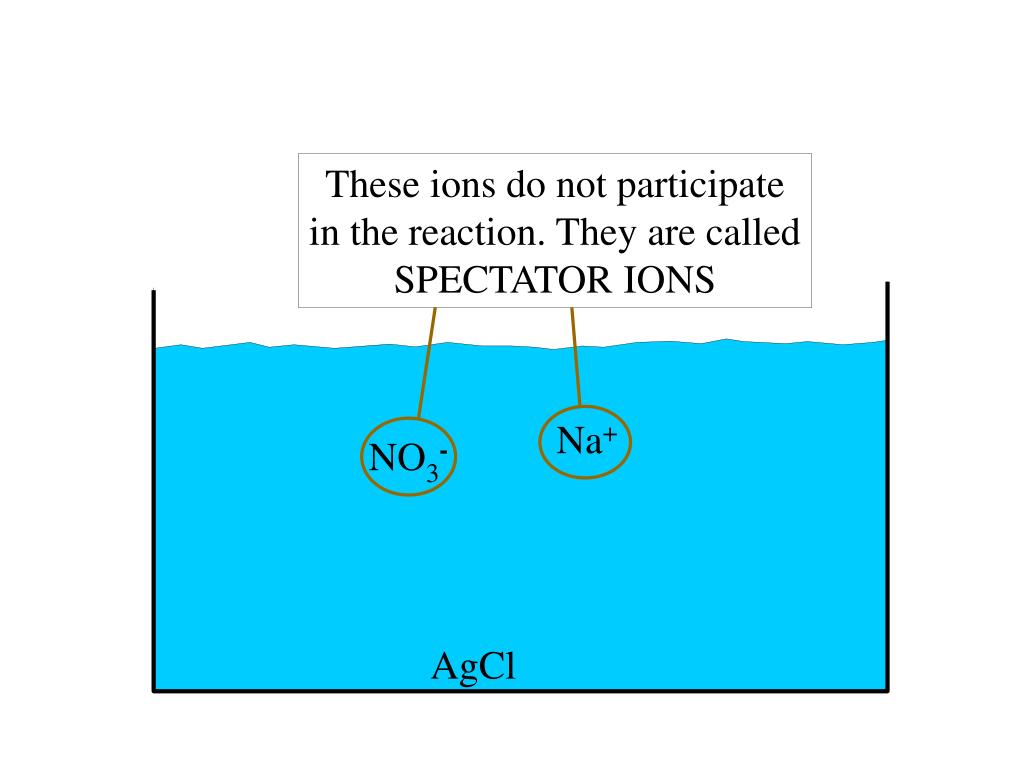
AgCl brought about out of resolution because it is insoluble in water. We first write all the ionic equation prior to we write the net ionic equation. To write the ionic equation, we separate all of the aqueous compounds into ions and depart the forged compound unseparated.
Ag+ + NO3- + Na+ + Cl- ---> AgCl(s) + Na+ + NO3-
Then, we fail to remember the spectator ions- the ions that appear on both sides of the equation. We're left now with the net ionic equation
Ag+ + Cl- ---> AgCl(s)
:)
Cl agno3, balancez agno3 + nacl = nano3 + agcl

PPT - AgNO 3 ( aq ) + NaCl( aq ) AgCl( s ) + NaNO 3 ( aq ...

PPT - Reaction Stoichiometry: Mole Method Calculations ...
Net Ionic Reaction Equations - Precipitation of AgCl 001 ...

PPT - Ch 17-18 Review PowerPoint Presentation, free ...

PPT - Chemical Reactions PowerPoint Presentation - ID:4011981
PPT - Net Ionic Equations PowerPoint Presentation, free ...
PPT - AgNO 3 ( aq ) + NaCl( aq ) AgCl( s ) + NaNO 3 ( aq ...
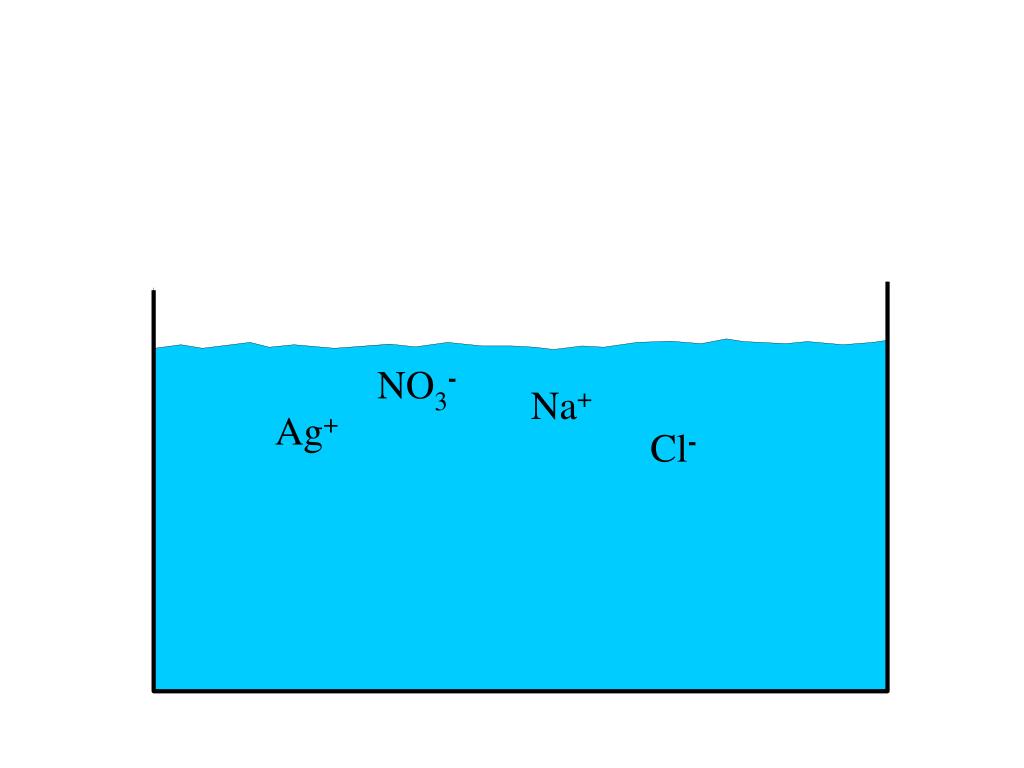
Chemistry - Chp 11 - Chemical Reactions - Notes

Cl agno3, balancez agno3 + nacl = nano3 + agcl

Net Ionic Equation Cake Ideas and Designs

PPT - AgNO 3 ( aq ) + NaCl( aq ) AgCl( s ) + NaNO 3 ( aq ...
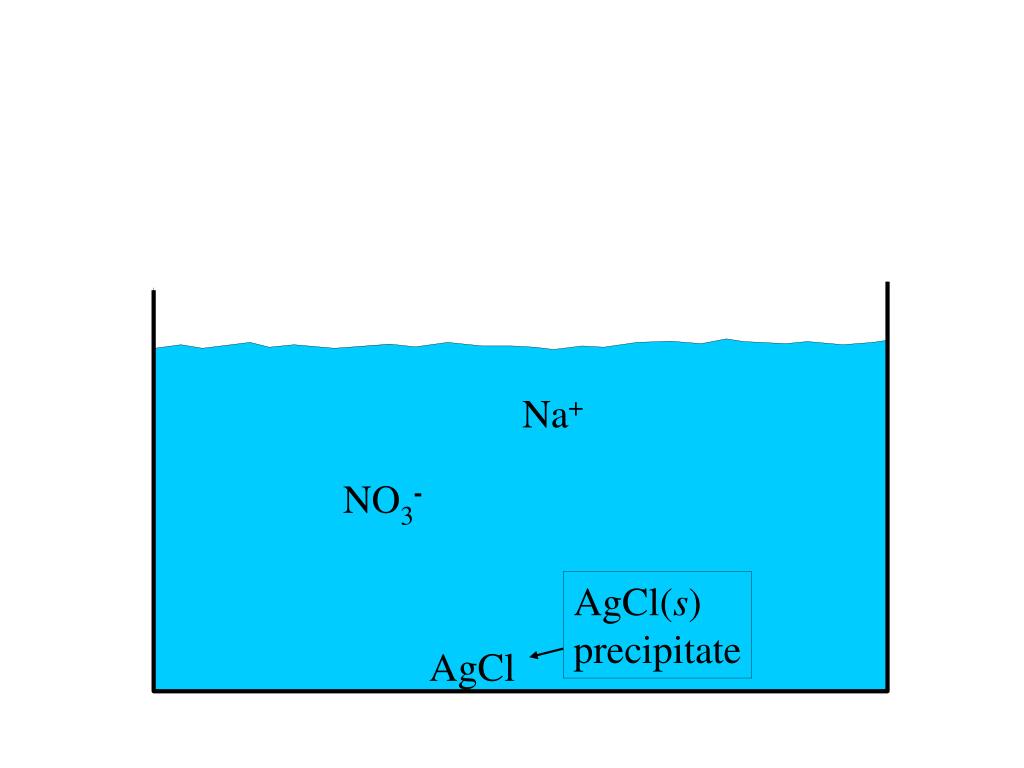
PPT - AgNO 3 ( aq ) + NaCl( aq ) AgCl( s ) + NaNO 3 ( aq ...
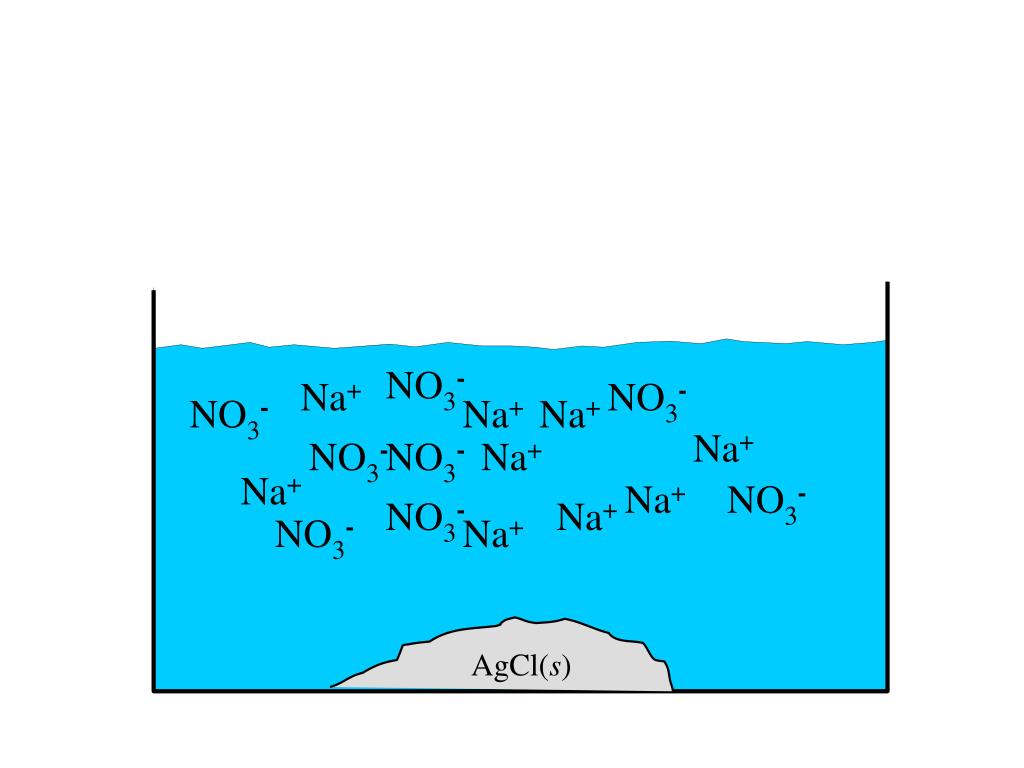
Chemistry - Chp 11 - Chemical Reactions - Notes

PPT - Reaction Stoichiometry: Mole Method Calculations ...

Solved: Write The Molecular And Net Ionic Equations, Inclu ...

PPT - Honors chemistry ch . 14: Ions in Aqueous Solutions ...

PPT - Chemistry 100(02) Fall 2010 PowerPoint Presentation ...
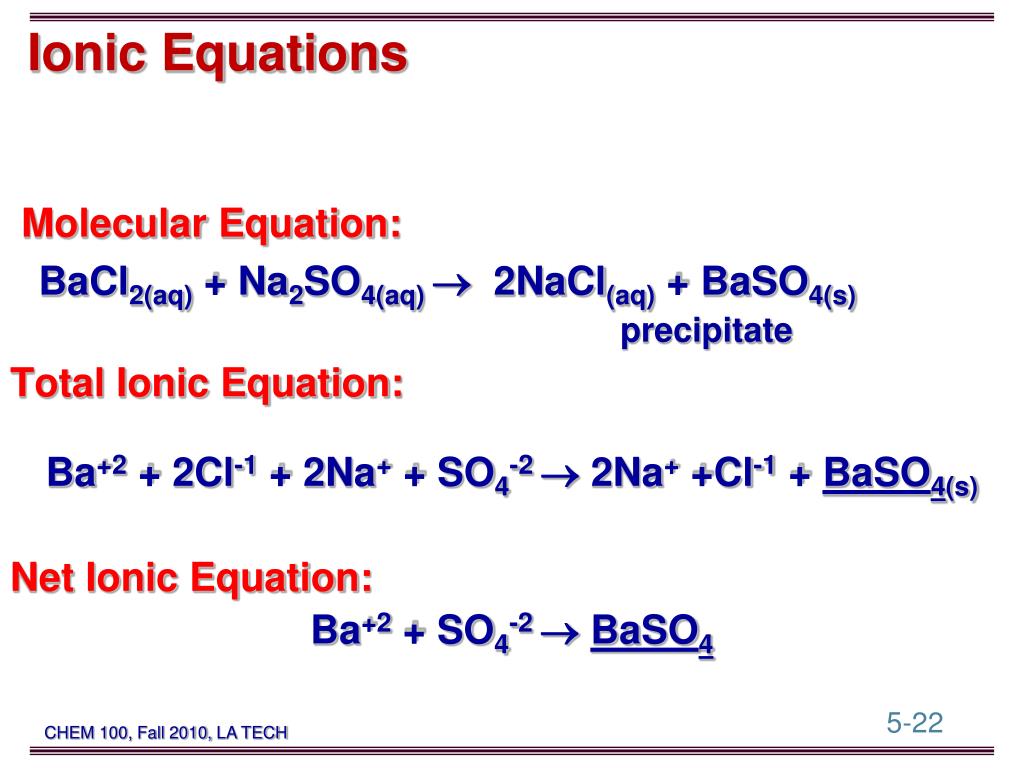
PPT - AgNO 3 ( aq ) + NaCl( aq ) AgCl( s ) + NaNO 3 ( aq ...
PPT - Ch 17-18 Review PowerPoint Presentation, free ...

PPT - ว 30131 เคมีพื้นฐาน พันธะเคมี PowerPoint ...
0 comments:
Post a Comment